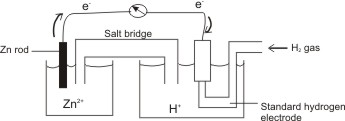
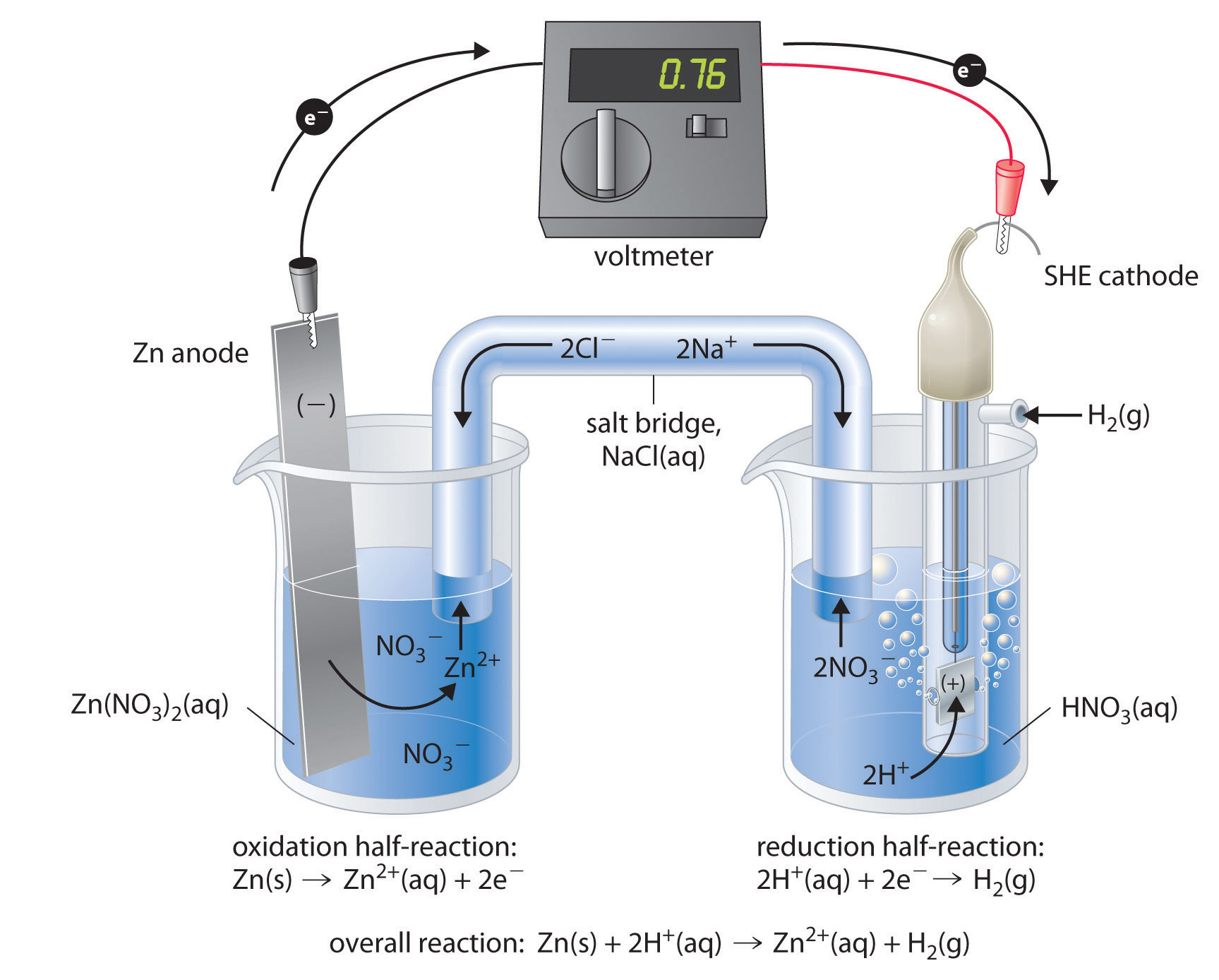
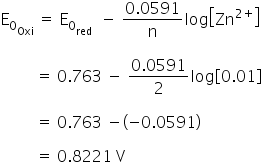The standard electrode potentials of Zn and Ni respectively are - 0.76 V and - 0.25 V. Then the standard emf of the spontaneous cell by coupling these under standard conditions is:

The standard reduction potentials of zinc, cadmium and copper are - 0.76 V, - 0.4 V and 0.34 V respectively. Select the correct statement.
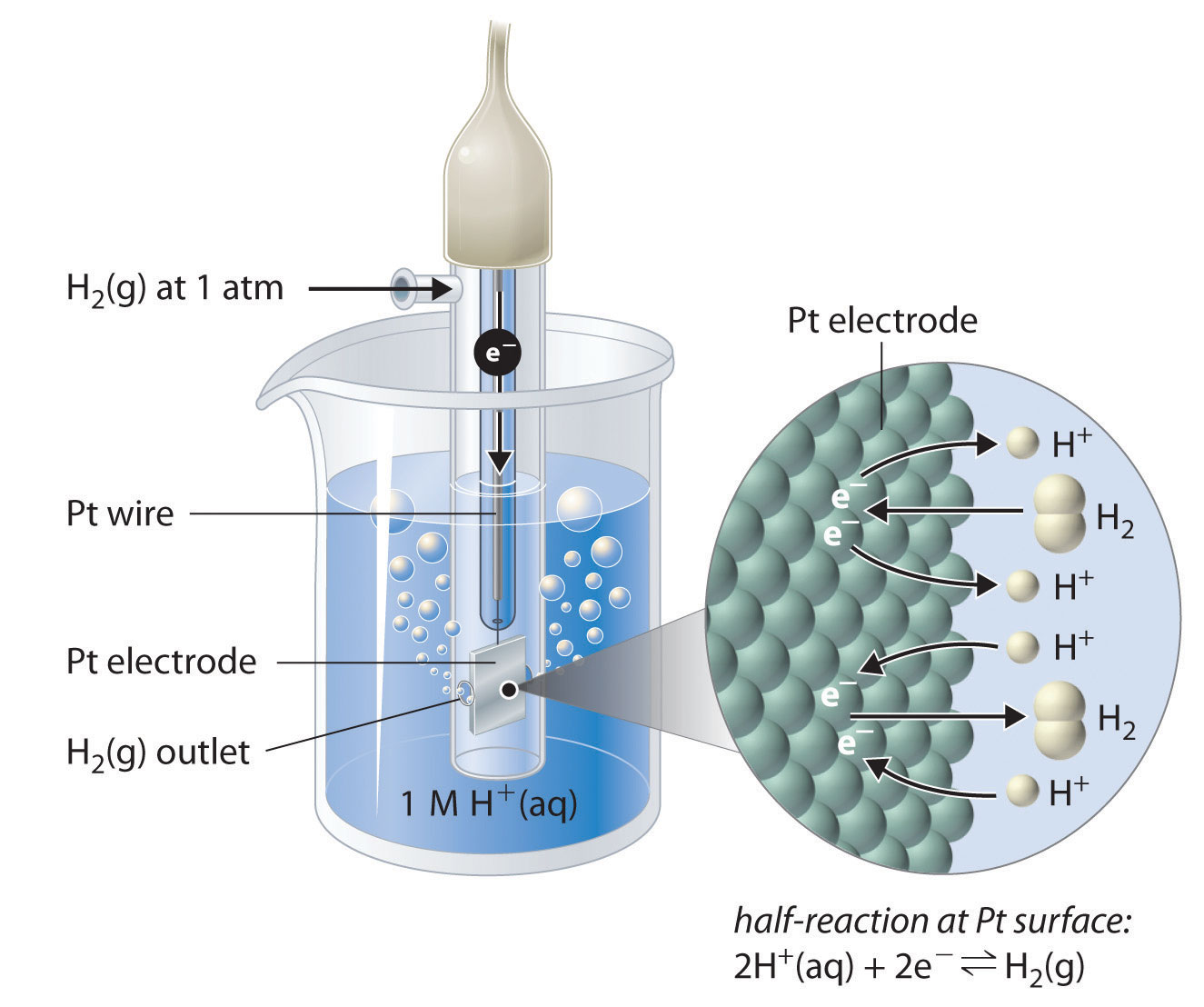
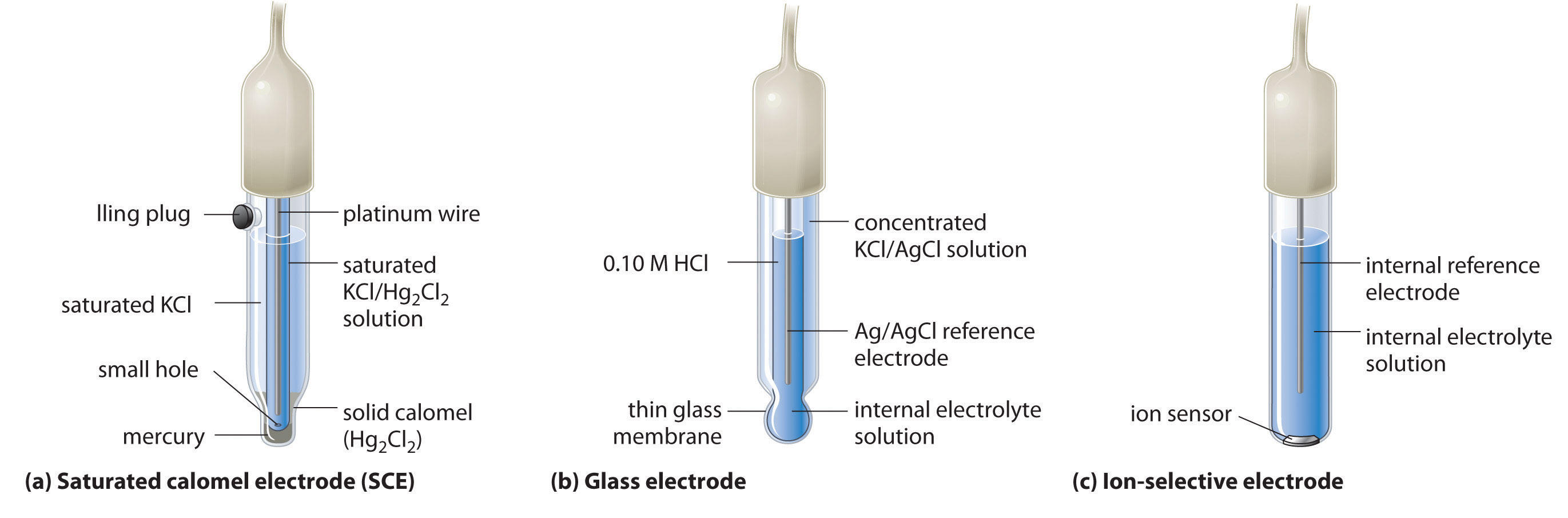
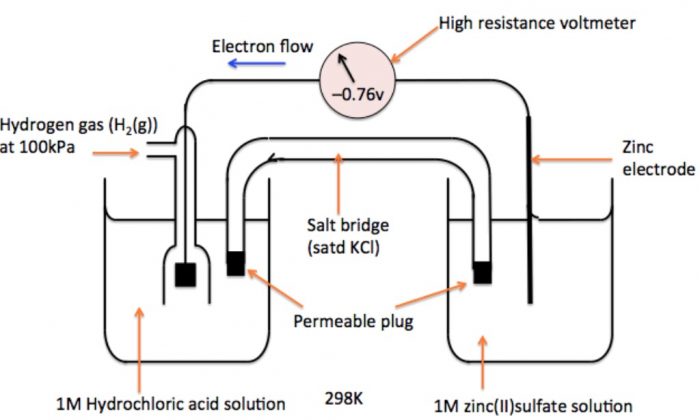
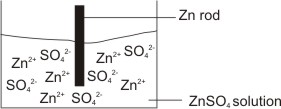
How to calculate the potential of zinc electrode capacity when in contact with 0.1M zinc sulphate solution in reference to hydrogen electrode when given the standard cell potential of Zn2 + /

The standard oxidation potential potential of zinc is 0.76 volt and of silver is - 0.80 volt. - YouTube

The standard reduction potentials of zinc, cadmium and copper are - 0.76 V, - 0.4 V and 0.34 V respectively. Select the correct statement.